Diphosphorus Pentoxide (s) + Water (l) Phosphoric Acid (aq)
How Many Lines Of Symmetry Does A Trapezoid Have, Lines of symmetry of an isosceles trapezium, 6.61 MB, 04:49, 2,448, Doubtnut, 2020-01-19T07:33:30.000000Z, 19, Properties of a trapezium or trapezoid (math facts) | Owlcation, hubpages.com, 1108 x 1200, jpeg, trapezium trapezoid symmetry lines properties many math does facts area line shape sides side holes parallel isosceles toppr pair questions, 20, how-many-lines-of-symmetry-does-a-trapezoid-have, KAMPION
For the following reaction, 3. 69 grams of water are mixed with excess diphosphorus pentoxide. The reaction yields 11. 5 grams of phosphoric acid. P2o5 (s) + 3h2o (l) 2h3po4 (aq) (1) what is the theoretical yield of phosphoric acid? ____grams (2) what is the percent yield for this reaction?
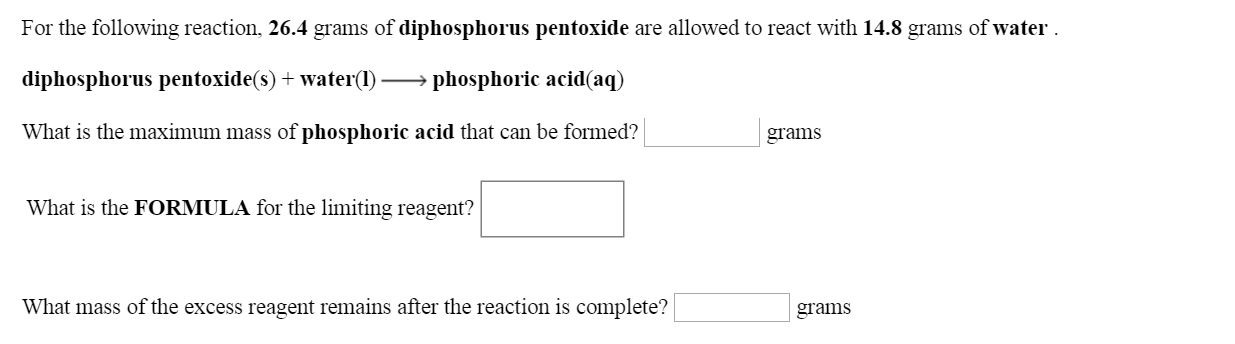
Diphosphorus pentoxide (s) + water (l) rightarrow phosphoric acid (aq) what is the maximum mass of phosphoric acid that can be formed? Grams what is the formula for the limiting reagent? What mass of the excess. For the following reaction, 31. 1 grams of diphosphorus pentoxide are allowed to react with with 10. 2 grams of water. Diphosphorus pentoxide (s) + water (l)>>> phosphoric acid (aq) what is the maximum amount of phosphoric acid that can be formed in grams? What is the formula for the limiting reagent? What is the balance equation for diphosphorus pentoxide and water? This problem has been solved! For the following reaction, 25. 5 grams of diphosphorus pentoxide are allowed to react with 13. 9 grams of water.
Solved: For The Following Reaction, 26.4 Grams Of Diphosph... | Chegg.com

Answered: For the following reaction, 5.21 grams… | bartleby
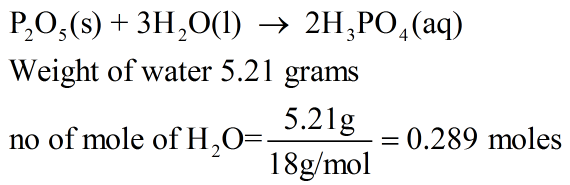
3 acid base reactions

Answered: According to the following reaction,… | bartleby

Answered: For the following reaction, 29.1 grams… | bartleby

Solved: For The Following Reaction, 29.0 Grams Of Diphosph... | Chegg.com

Solved: According To The Following Reaction, How Many Gram... | Chegg.com

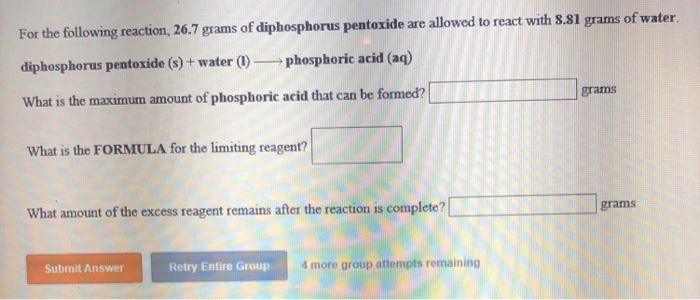
Solved: For The Following Reaction, 26.7 Grams Of Diphosph... | Chegg.com
For this reaction, 31.8 g diphosphorus pentoxide | Chegg.com

Komentar
Posting Komentar