How Many Electrons Are There In The 2nd Principal Energy Level (n = 2) Of A Phosphorus Atom?
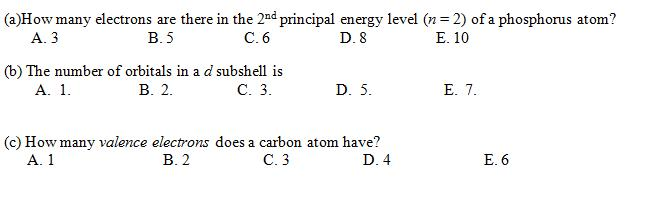
How Many Lines Of Symmetry Does A Trapezoid Have, Lines of symmetry of an isosceles trapezium, 6.61 MB, 04:49, 2,448, Doubtnut, 2020-01-19T07:33:30.000000Z, 19, Properties of a trapezium or trapezoid (math facts) | Owlcation, hubpages.com, 1108 x 1200, jpeg, trapezium trapezoid symmetry lines properties many math does facts area line shape sides side holes parallel isosceles toppr pair questions, 20, how-many-lines-of-symmetry-does-a-trapezoid-have, KAMPION
How many electrons are there in the 2nd principal energy level n 2 of an aluminum atom? Eight electrons aluminum has 13 electrons so it will have the electron arrangement (2, 8, 3) which represents two electrons in the n=1 energy level, eight electrons in the n=2 level, and three electrons in the n=3 level. Click here 👆 to get an answer to your question ️ how many electrons are there in second principal energy level(n=2) of a phosphorus. A neutral phosphorus atom has 15 electrons.
That le… bakhora03 bakhora03 4 days ago chemistry college answered how many electrons are there in the principal energy level (n=2) of a phosphorus atom? The formula defining the energy levels of a hydrogen atom are given by the equation: The energy is expressed as a negative number because it takes that much energy to unbind (ionize) the electron from the nucleus.
Solved: Principal Energy Levels, Orbitals, Valence Electro... | Chegg.com
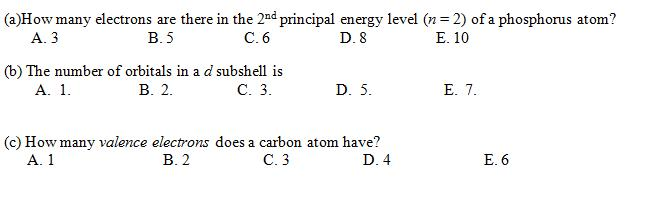
Solved: Which Orbital Are These Electrons In And Number Of... | Chegg.com
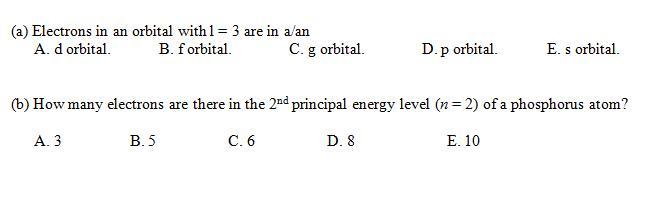
Komentar
Posting Komentar