Why Does Atomic Size Increase Down A Group

How Many Lines Of Symmetry Does A Trapezoid Have, Lines of symmetry of an isosceles trapezium, 6.61 MB, 04:49, 2,448, Doubtnut, 2020-01-19T07:33:30.000000Z, 19, Properties of a trapezium or trapezoid (math facts) | Owlcation, hubpages.com, 1108 x 1200, jpeg, trapezium trapezoid symmetry lines properties many math does facts area line shape sides side holes parallel isosceles toppr pair questions, 20, how-many-lines-of-symmetry-does-a-trapezoid-have, KAMPION
The atomic size increases from top to bottom in a group as the atomic number increases. Since there are more filled energy levels so the valence electrons are found farther from the nucleus increasing the atomic size. On moving down the group, new shells are being added. The nuclear charge is not enough to reduce the size enough.
The relative atomic mass increases as you go down a group because as you go down a group, not only does the atomic number increase, but so does the atomic. You would also expect that going down a group, nuclear charge increases (more protons), however, the electrostatic strength down a group is relatively the same. Well because there are more shielding due to more energy levels so overall nuclear attraction is similar. Z eff = z(no. Similarly, why does atomic size increase down a group and decrease left to right? Down a group, atomic radius increases. atomic radius decreases from left to right within a period. This is caused by the increase in the number of protons and electrons across a period. One proton has a greater effect than one electron; Thus, electrons are pulled towards the nucleus, resulting in a smaller.
As an atoms radius decreases > MISHKANET.COM

PPT - History of the Periodic Table PowerPoint Presentation - ID:1210134
Trends of periodic atomic properties
PPT - Atoms PowerPoint Presentation, free download - ID:2682421

Periodic Relationships: May 2012

PPT - Periodic Trends PowerPoint Presentation, free download - ID:3652853
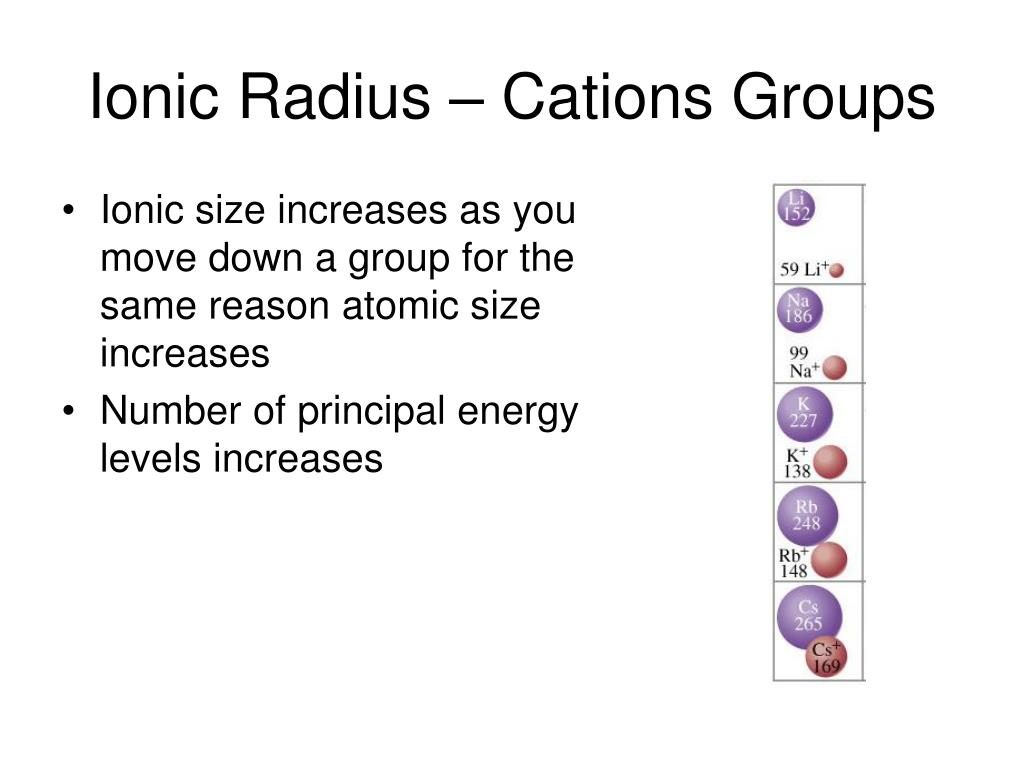
Periodic Behavior - Presentation Chemistry
Why Does Atomic Radius Decrease Across A Period - slidedocnow
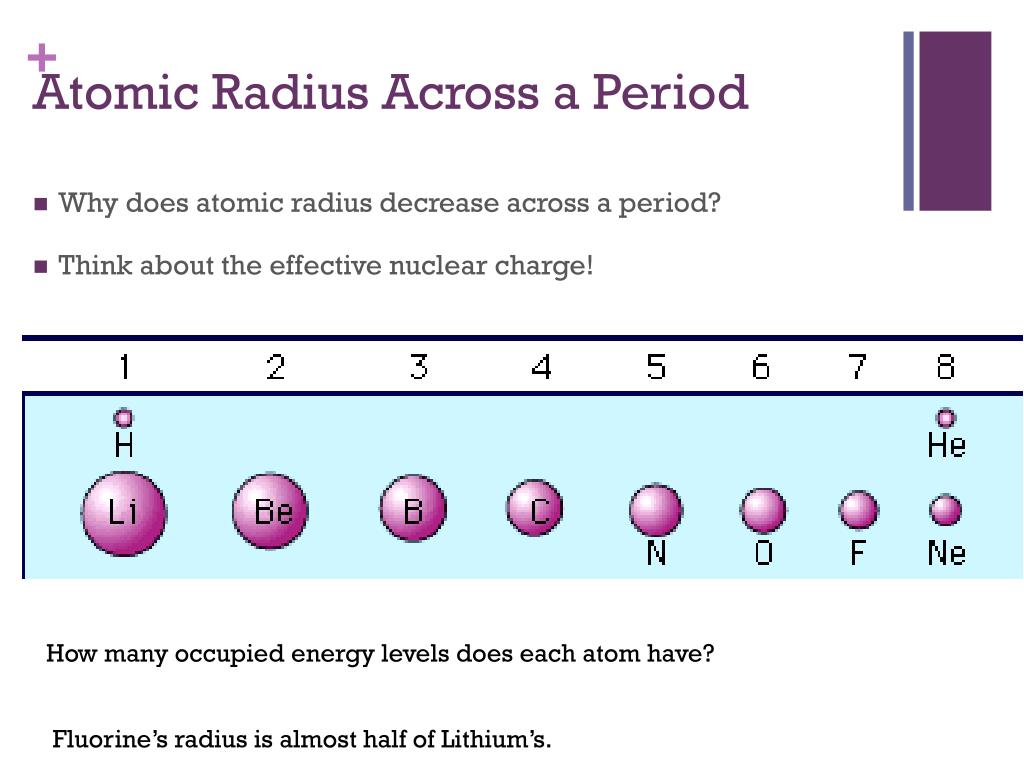
Trends in the periodic table
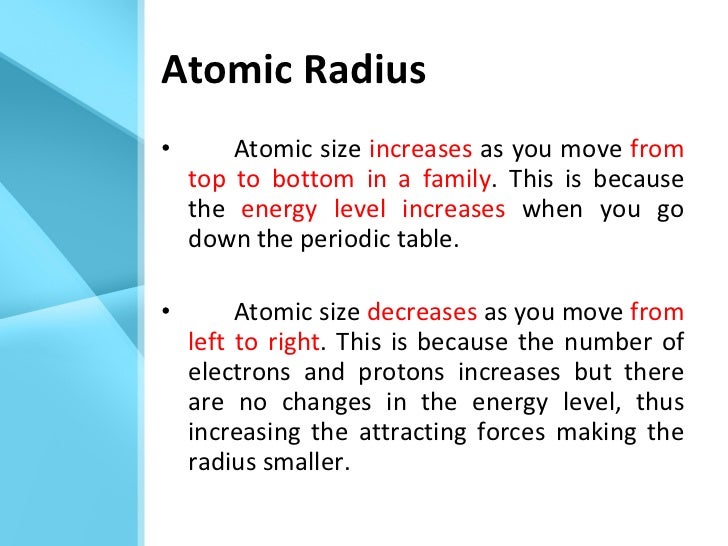
Komentar
Posting Komentar