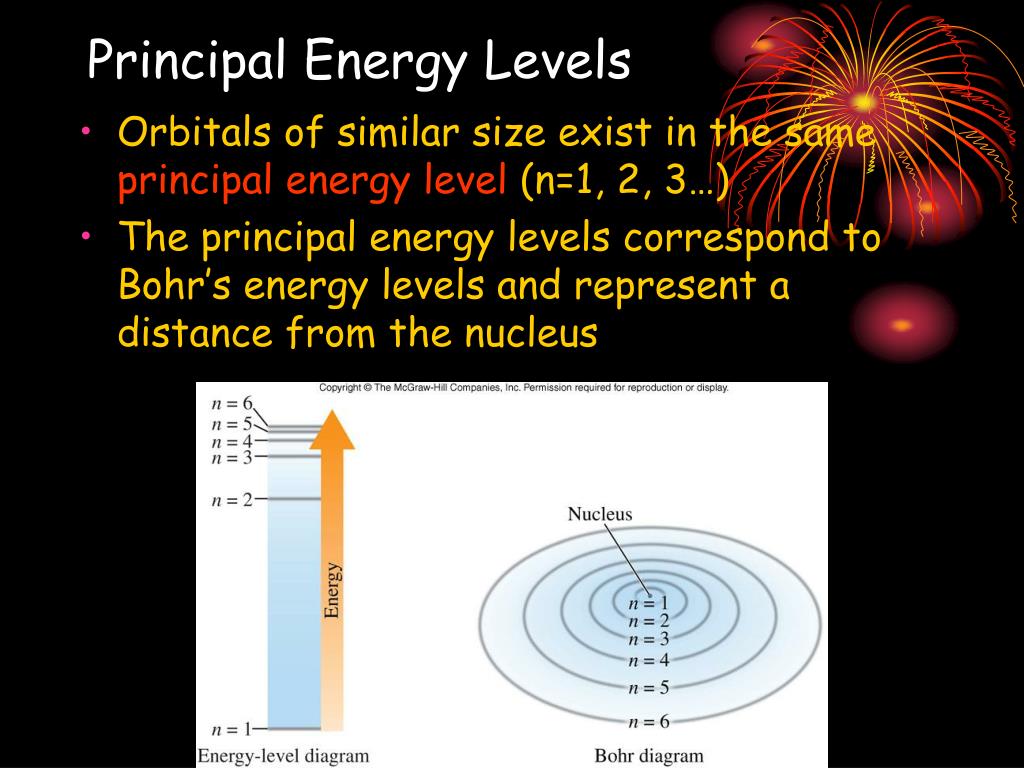
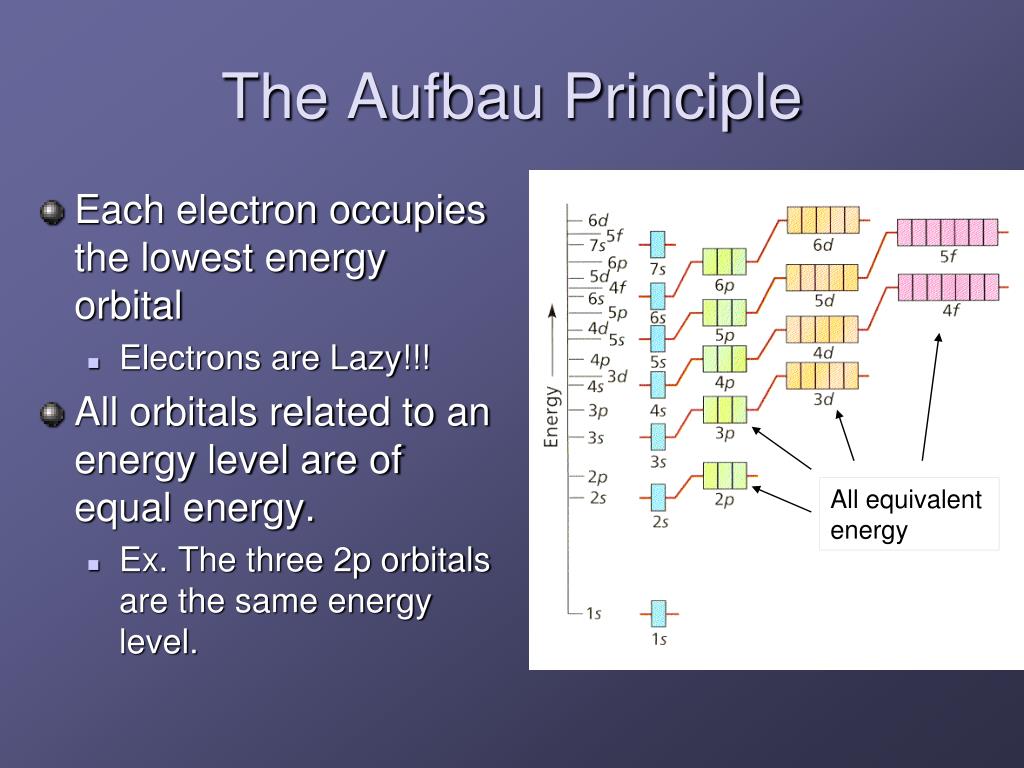
What Is The Lowest Energy Level That Contains D Orbitals
How Many Lines Of Symmetry Does A Trapezoid Have, Lines of symmetry of an isosceles trapezium, 6.61 MB, 04:49, 2,448, Doubtnut, 2020-01-19T07:33:30.000000Z, 19, Properties of a trapezium or trapezoid (math facts) | Owlcation, hubpages.com, 1108 x 1200, jpeg, trapezium trapezoid symmetry lines properties many math does facts area line shape sides side holes parallel isosceles toppr pair questions, 20, how-many-lines-of-symmetry-does-a-trapezoid-have, KAMPION
It contains all kinds of stuff that the body can’t. The rest of the body can’t store energy without the brain, so the brain is the only place where we store our energy. For most people, the lowest energy level that contains d orbitals is. What is the lowest energy level that contains d orbitals?
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p. 14 what is a main energy level. 15 what is the second energy level of an. The lowest energy level that has f orbitals is the fourth energy level. The atomic orbital of any atom only contains 2 electrons. Which is the lowest energy level that contains d orbitals? Be notified when an answer is posted.
WEEK 3: CHEMISTRY 111 – Chapter 9 – Electron Structure
Atoms and Atomic Structure | HubPages

PPT - Chapter 4 Electron Structure of the Atom PowerPoint Presentation
PPT - Electron Configuration! PowerPoint Presentation, free download
PPT - Electron configurations PowerPoint Presentation, free download

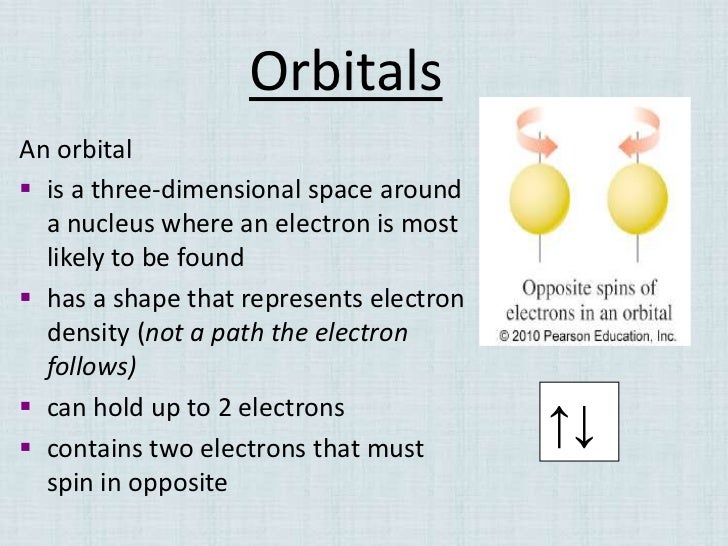
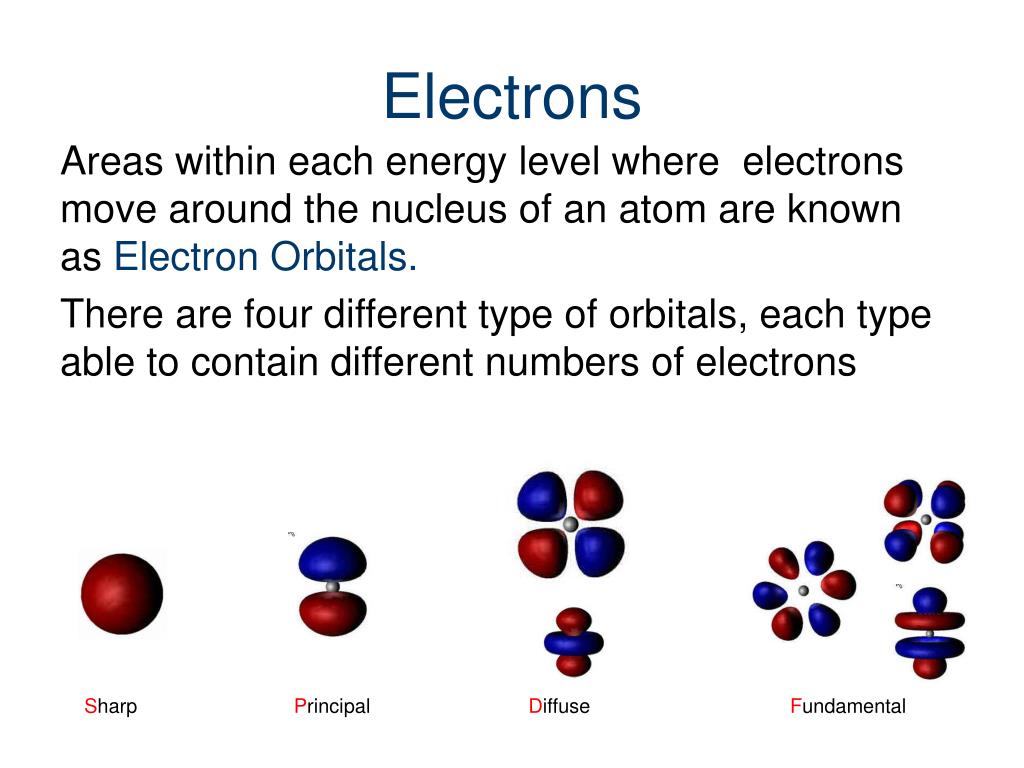
Chemistry: Electron orbitals and sub levels
Chemistry: Electron orbitals and sub levels
PPT - Atomic Structure PowerPoint Presentation, free download - ID:1680708
PPT - Chapter 12 Electrons in Atoms PowerPoint Presentation, free
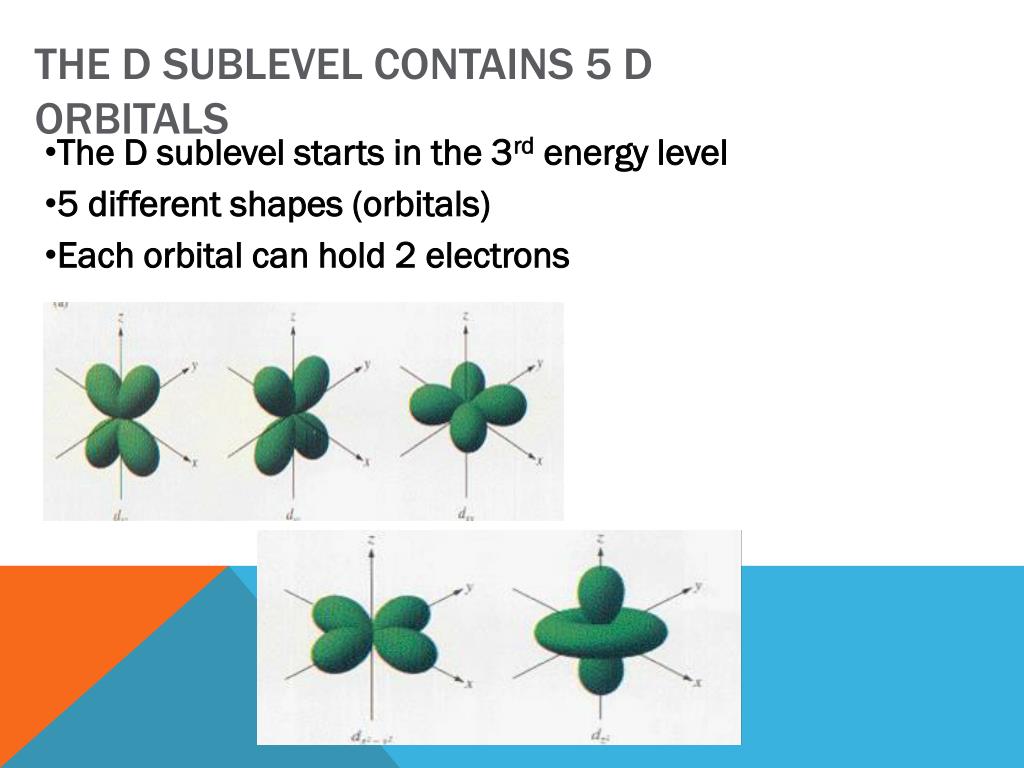
PPT - Electron configurations PowerPoint Presentation, free download
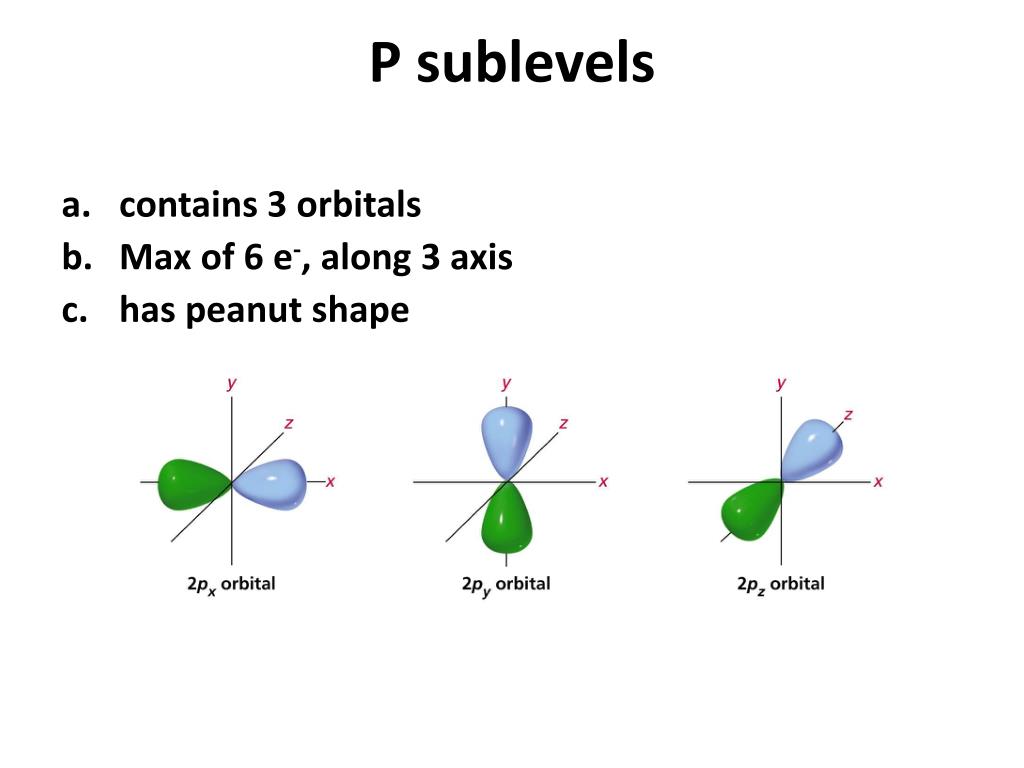
Komentar
Posting Komentar